Iron is a metallic element that participates in chemical reactions important for biological processes. The physiological role of iron is as a component of heme and in other protein functional groups (Dev and Babitt, 2017). It is generally found in two different ionic forms: Fe2+, the reduced form and Fe3+, the oxidized form (Dev and Babitt, 2017). In excess, iron in the Fe2+ form can lead to the production of toxic free radicals that can damage cell structures; it is thus highly regulated in the body (Vogt et al, 2021).
Iron is brought into the body through the diet. The estimated average requirement for iron intake is 5 to 11.4 mg/day (Lim et al, 2013). Meat, seafood, legumes and fortified cereals are all significant dietary sources of iron (Lim et al, 2013). Although plant-derived foods contain slightly more iron on average than animal-derived foods, iron from heme in animal tissues is more bioavailable — more able to be absorbed and requiring less energy to be useful in its physiological role (Lim et al, 2013). Phytates (a form of phosphate storage in seeds and grains) and polyphenols (antioxidants found in tea and coffee) reduce the bioavailability of iron. Vitamin C, on the other hand, increases bioavailability (Lim et al, 2013).
In the digestive tract, iron is taken up by the cells of the duodenum. It must be in the Fe2+ form for this to happen, so iron that is in the Fe3+ form is converted by the cytochrome DCYTB (Vogt et al, 2021). Fe2+ ions enter duodenal cells through the transport protein DMT1. The iron can then be sent to the bloodstream by the transport protein ferroportin on the other side of the cell. It is immediately converted back to Fe3+ by the proteins ceruloplasmin and hephaestin and binds to transferrin, a carrier protein in the bloodstream (Vogt et al, 2021).
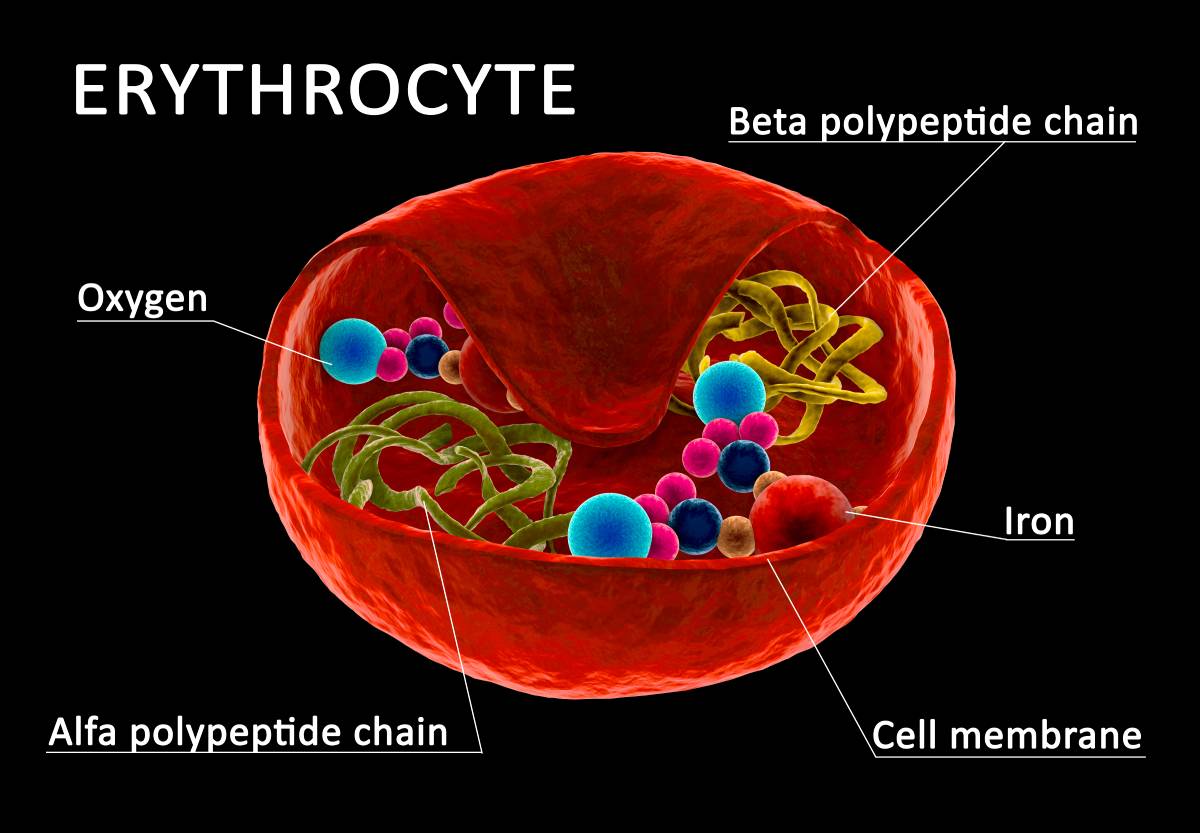
The most common physiological role of iron in the body is for hemoglobin in red blood cells, also known as erythrocytes (Vogt et al, 2021). Erythroid precursor cells have transferrin receptor 1. When transferrin binds to the receptor, the complex is internalized by the cell. The Fe3+ that was bound to the transferrin is released and is turned back into Fe2+ by protein STEAP3. The iron is finally available to be incorporated into heme (Vogt et al, 2021). A similar process occurs in the muscle cells for the production of myoglobin, which allows muscles to maintain their own small stores of oxygen. Cells can also store iron that they intake with the intracellular protein ferritin. Cells are able to regulate their iron storage through how many ferritin proteins and transferrin receptors they make (Vogt et al, 2021).
Systemically, iron levels are regulated by the liver. The liver produces the hormone hepcidin, which causes ferroportin to be destroyed. Doing this prevents excess iron from entering circulation (Dev and Babitt, 2017). Additionally, immune system macrophages contribute to iron regulation by recycling iron stores. Once the macrophages have cleaned up an old or damaged red blood cell, they can remove the iron from the hemoglobin and use ferroportin to transport it back into the bloodstream (Dev and Babitt, 2017).
Problems with iron homeostasis can lead to health complications. One example is anemia, which is a problem with red blood cells’ ability to carry oxygen. Iron deficiency anemia can occur when there is not enough dietary iron available; excessive iron loss, such as bleeding or chronic kidney disease; genetic issues with any of the proteins mentioned previously; or chronic immune conditions that cause an overproduction of hepcidin (Dev and Babitt, 2017). On the other hand, iron overload can cause highly reactive forms of iron to be taken up by organs such as the heart, liver and endocrine tissues and cause lasting damage and dysfunction (Dev and Babitt, 2017).
References
Dev S, Babitt JL. Overview of iron metabolism in health and disease. Hemodial Int. 2017;21 Suppl 1(Suppl 1):S6-S20. doi:10.1111/hdi.12542
Lim KH, Riddell LJ, Nowson CA, Booth AO, Szymlek-Gay EA. Iron and zinc nutrition in the economically-developed world: a review. Nutrients. 2013;5(8):3184-3211. Published 2013 Aug 13. doi:10.3390/nu5083184
Vogt AS, Arsiwala T, Mohsen M, Vogel M, Manolova V, Bachmann MF. On Iron Metabolism and Its Regulation. Int J Mol Sci. 2021;22(9):4591. Published 2021 Apr 27. doi:10.3390/ijms22094591